The Stephanopoulos lab at MIT is actively involved in engineering a microbial system for the production of biofuels in the form of fatty acid methyl esters (FAMEs), a renewable energy source for transportation that can be derived from triacylglycerides (TAGs), naturally produced within cells. Our goal is to engineer a host organism such that it can convert renewable feedstocks into intracellular lipids with high titer, productivity, and yield. The oleaginous yeast Yarrowia lipolytica has been of primary interest in the lab because of its ability to accumulate large quantities of TAGs, tolerate a wide range of conditions, and utilize many different substrates. In addition, the availability of genetic tools and sequencing information enables us to modify the organism metabolically to further enhance its lipid production features. Currently, our lab focuses on establishing strains capable of economic production of diesel and jet fuel using either sugars or volatile fatty acids (VFAs) as carbon substrates.
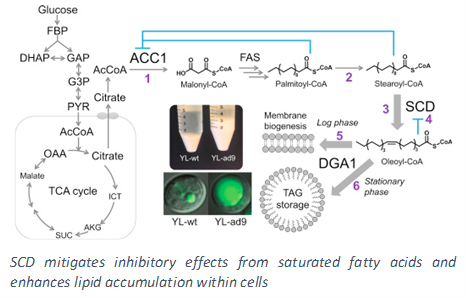
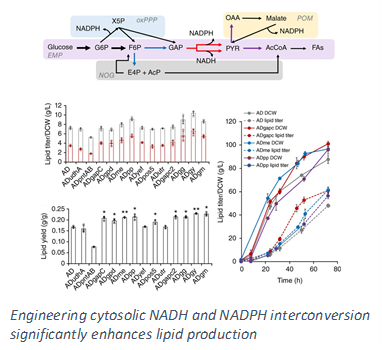
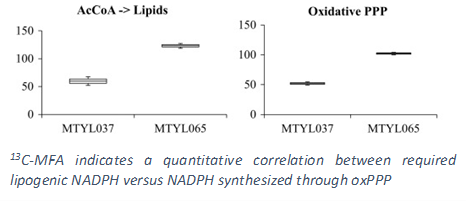
To accomplish the above goals, our lab uses a rational engineering approach in which we first identify the bottlenecks within the metabolic network that hinder lipid overproduction and then carry out genetic alterations that overcome such bottlenecks. Early efforts in identification of rate limiting steps involved the discovery of key regulatory and inhibitory effects of the lipid synthesis enzymes. In particular, the accumulation of long chain free fatty acids, a metabolic precursor in triacylglyceride formation, inhibits overall synthesis. Furthermore, saturated fatty acids have more of an inhibitory effect compared to that of unsaturated fatty acids. More recently we have shifted our attention to employing 13C Metabolic Flux Analysis (MFA), which we have pioneered in our lab, to systematically analyze the entire metabolic network such that a global understanding of the key pathways involved in lipid production can be obtained. Our results have helped us identify that the limiting factor for lipid synthesis in Y. lipolytica is the availability of cytosolic NADPH, a critical reducing cofactor. We have also demonstrated that the lipogenic NADPH is supplied primarily from the oxidative pentose phosphate pathway (PPP).
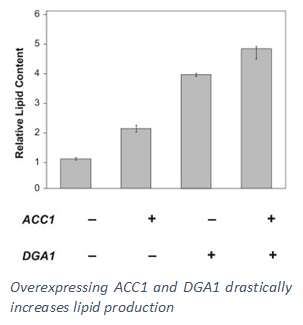
Guided by these findings, our engineering efforts for debottlenecking lipid synthesis in Y. lipolytica have explored overexpression of the enzyme responsible for the incorporation of fatty acids into triacyglycerides (DGA1), thereby reducing the level of free fatty acids in the cytosol. In addition, a mammalian cell enzyme capable to efficiently converting saturated fatty acids into unsaturated fatty acids (SCD) was expressed in Y. lipolytica to further mitigate the inhibitory effects. The strain was successfully tested in large scale bioreactors achieving very high lipid titer and productivity. In order to provide additional cytosolic NADPH, we have also designed and implemented synthetic pathways to recycle excess cytosolic NADH and convert it into NADPH needed for lipid production. This has not only further enhanced the lipid accumulation capacity of the organism but also increased the yield close to the theoretical maximum, pushing the overall process closer to economic production.
Key Publications
Xu, J., Liu, N., Qiao, K., Vogg, S., Stephanopoulos, G., 2017. Application of metabolic controls for the maximization of lipid production in semicontinuous fermentation. Proceedings of the National Academy of Sciences, doi: 10.1073/pnas.1703321114
Qiao, K., Wasylenko, T.M., Zhou, K., Xu, P. & Stephanopoulos G. 2016. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nature Biotechnology, doi:10.1038/nbt.3763
Liu, N., Qiao, K., Stephanopoulos, G. 13C Metabolic Flux Analysis of acetate conversion of lipids by Yarrowia lipolytica. Metabolic Engineering. 38, pp.86-97
Wasylenko, T.M., Ahn, W.S., Stephanopoulos, G., 2015. The oxidative pentose phosphate pathway is the primary source of NADPH for Lipid Overproduction from Glucose in Yarrowia lipolytica. Metabolic Engineering, 30, pp.27–39.
Tai, M., Stephanopoulos, G., 2013. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metabolic Engineering, 15(1), pp.1–9