Engineering the fermentation yeast Saccharomyces cerevisiae for the renewable production of biofuels and industrial biocommodities has spanned three major areas:
Tolerance — Cellular toxicity to substrates and/or products in the fermentation medium remains one of the most complex and elemental physiological constraints limiting higher production. Even in the simple case of ethanol (the native metabolic product for yeast), high concentrations trigger cell death via biological mechanisms that have long remained elusive. We have taken both rational and inverse approaches to enhancing such a genetically intricate phenotype.
Strengthening membrane electrochemical gradients – We have uncovered a fundamental mechanism of general alcohol toxicity whereby alcohols impinge viability not by solubilizing lipid bilayers, but by increasing plasma membrane permeability and dissipating the cell’s critical membrane potential. Since the yeast membrane is charged predominantly by opposing gradients of potassium (K+) and proton (H+) ions, elevating extracellular K+ and pH counteract these dissipating effects and significantly enhance tolerance. These simple modifications boost ethanol titers into the 100-150 g/L range universally in laboratory and commercial strains, as well as increase viability to higher energy — and more toxic — alcohols such as isobutanol. Likewise, specific up-regulation of the membrane K+ and H+ pumps transforms underperforming laboratory strains to ones surpassing current industrial strains.

Global transcription machinery engineering (gTME) – Given that ethanol likely attacks multiple cellular targets, we have developed a genome-wide perturbation and selection technique for eliciting superior tolerance phenotypes. By introducing mutated alleles of SPT15 — a component of the general transcription pre-initiation complex — into yeast, hundreds of transcripts can be modulated simultaneously to significantly re-program stress homeostasis. Subjecting these yeast libraries to increasing ethanol concentrations subsequently allows strains containing transcriptomes conferring the highest tolerance to be isolated. In addition to a strain exhibiting 4x improvement in growth, we identified SAGA-mediated pathways as potential mechanisms the cell induces to combat ethanol stress.
Feedstocks — Successfully exploiting next-generation feedstocks based on lignocellulosic biomass requires that yeast be endowed with new capabilities. Primary among these is the ability to consume xylose, a sugar that yeast cannot metabolize natively and of which lignocellulose contains a significant proportion. To address this issue, we have created strains capable of converting xylose to ethanol using a combination of rational and evolutionary methods. Expression of the XYLA and XYL3 genes (from Piromyces and S. stipitis, respectively) enable yeast to assimilate xylose into the pentose phosphate pathway. Further engineering of the non-oxidative portion of the pentose phosphate pathway, followed by adaptive evolution under xylose-limited conditions, produced a strain exhibiting one of the highest xylose consumption rates and xylose-to-ethanol yields reported.
Since the cost-effective pretreatment of lignocellulose into fermentable sugars releases numerous compounds inhibitory to yeast, cellular tolerance is as much a feedstock issue as a product issue. The most significant of these inhibitors are furan aldehydes and organic acids which are present in amounts that impact viability and potentially exacerbate ethanol damage. Given the nature of this combinatorial toxicity, we are exploring tolerance strategies that involve the techniques described above as well as those involving compound-specific mitigation.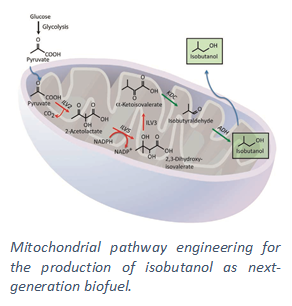
Products — To expand beyond ethanol to next-generation renewable fuels and chemical commodities, we are engineering yeast with heterologous pathways for the synthesis of several important non-native products. While traditional metabolic engineering has enabled basic expression of these pathways, efforts to leverage eukaryotic physiology have led us to develop organelle engineering as a strategy to increase product output. For example, synthesis of the next-generation biofuel isobutanol has been demonstrated in bacteria and can be achieved by five enzymatic conversions from the glycolytic end-product pyruvate. However, by exploiting subcellular compartmentalization, we increased titers up to 5x predominantly by localizing these steps into mitochondria where key intermediates are concentrated.
Key Publications
A.J. Shaw, F.H. Lam, M. Hamilton, A. Consiglio, K. MacEwen, E.E. Brevnova, E. Greenhagen, W.G. LaTouf, C.R. South, H. van Dijken, G. Stephanopoulos. (2016) Metabolic engineering of microbial competitive advantage for industrial fermentation processes. Science. 353, 583–586.
M. Wasylenko, G. Stephanopoulos, Metabolomic and 13C-metabolic flux analysis of a xylose-consuming Saccharomyces cerevisiae strain expressing xylose isomerase. Biotechnol Bioeng. 112, 470–483 (2015).
H. Lam, A. Ghaderi, G. R. Fink, G. Stephanopoulos, Engineering alcohol tolerance in yeast. Science. 346, 71–75 (2014).
L. Avalos, G. R. Fink, G. Stephanopoulos, Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols. Nat Biotechnol. 31, 335–341 (2013).
Zhou, J.-S. Cheng, B. L. Wang, G. R. Fink, G. Stephanopoulos, Xylose isomerase overexpression along with engineering of the pentose phosphate pathway and evolutionary engineering enable rapid xylose utilization and ethanol production by Saccharomyces cerevisiae. Metab Eng. 14, 611–622 (2012).
Alper, J. Moxley, E. Nevoigt, G. R. Fink, G. Stephanopoulos, Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 314, 1565–1568 (2006).