 Isoprenoids are a large and important class of secondary metabolites, with more than 55,000 currently known. Naturally, these molecules play physiological roles in maintaining membrane fluidity (sterols and archeal membrane substituents), as hormones (estrogen, giberellin), photosynthetic pigments (chlorophyll, carotenoids), vitamins (A, D, E, and K), in plant chemical defense (phytoalexin), and, as attractants for pollinators and seed dispersal. However, these molecules have also been co-opted for use by humans and are now ubiquitous in daily life. Isoprenoid flavors and fragrances are a large constituent in perfumes, shampoos, and cleaning products, and other uses range from the citronellol in insect repellant to natural rubber in tires, as well as nutritional supplements and lifesaving pharmaceuticals such as the anti-malarial drug Artemesin and the chemotherapeutic agent Taxol.
Isoprenoids are a large and important class of secondary metabolites, with more than 55,000 currently known. Naturally, these molecules play physiological roles in maintaining membrane fluidity (sterols and archeal membrane substituents), as hormones (estrogen, giberellin), photosynthetic pigments (chlorophyll, carotenoids), vitamins (A, D, E, and K), in plant chemical defense (phytoalexin), and, as attractants for pollinators and seed dispersal. However, these molecules have also been co-opted for use by humans and are now ubiquitous in daily life. Isoprenoid flavors and fragrances are a large constituent in perfumes, shampoos, and cleaning products, and other uses range from the citronellol in insect repellant to natural rubber in tires, as well as nutritional supplements and lifesaving pharmaceuticals such as the anti-malarial drug Artemesin and the chemotherapeutic agent Taxol.
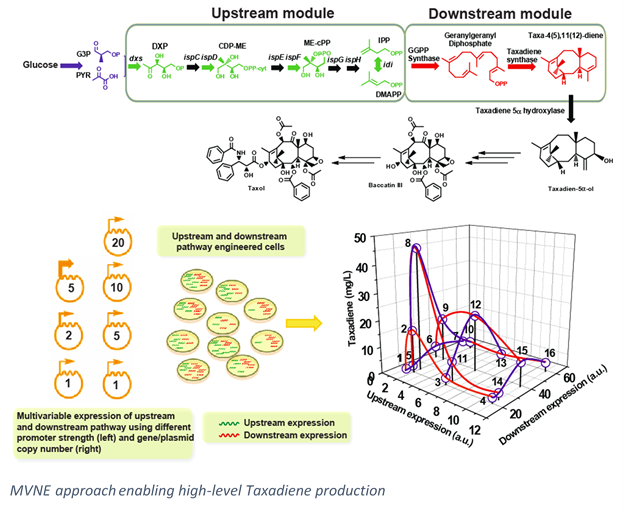
Our laboratory applies metabolic engineering and synthetic biology to understand and synthesize these 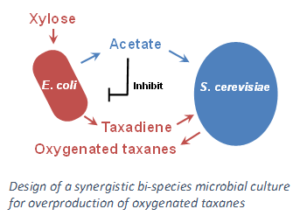 molecules at industrially-relevant levels. We focus both on the upstream pathway, responsible for supplying the building blocks for all isoprenoids (IPP and DMAPP), primarily through the microbial 2-methyl-(D)-erythritol-4-phosphate pathway (MEP pathway). This is performed though methods such as multivariate modular engineering of pathways to find the optimal expression levels of pathway genes and through the use of novel arrangements of co-cultured organisms. These strategies, among others, have enabled our lab to create platforms for high level production of isoprenoids.
molecules at industrially-relevant levels. We focus both on the upstream pathway, responsible for supplying the building blocks for all isoprenoids (IPP and DMAPP), primarily through the microbial 2-methyl-(D)-erythritol-4-phosphate pathway (MEP pathway). This is performed though methods such as multivariate modular engineering of pathways to find the optimal expression levels of pathway genes and through the use of novel arrangements of co-cultured organisms. These strategies, among others, have enabled our lab to create platforms for high level production of isoprenoids.
We also focus on production of individual isoprenoids, with a large focus on heterologous production of the anticancer drug Taxol (Paclitaxel). We have successfully produced gram-scale titers of the first cyclized precursor, and have performed enzymatic studies to revealed previously unknown bottlenecks and methods by which these bottlenecks may be alleviated.
Key Publications:
Edgar, Steven, et al. “Mechanistic Insights into Taxadiene Epoxidation by Taxadiene-5α-Hydroxylase.” ACS chemical biology 11.2 (2015): 460-469.
Zhou, Kang, et al. “Distributing a metabolic pathway among a microbial consortium enhances production of natural products.” Nature biotechnology 33.4 (2015): 377-383.
Ajikumar, Parayil Kumaran, et al. “Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli.” Science 330.6000 (2010): 70-74.
Alper, Hal, Kohei Miyaoku, and Gregory Stephanopoulos. “Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets.” Nature biotechnology 23.5 (2005): 612-616.